Green-backed Trogon Trogon viridis Scientific name definitions
- LC Least Concern
- Names (24)
- Monotypic
Text last updated May 7, 2015
Sign in to see your badges
Species names in all available languages
| Language | Common name |
|---|---|
| Catalan | trogon dorsiverd |
| Czech | trogon zelenohřbetý |
| Dutch | Groenrugtrogon |
| English | Green-backed Trogon |
| English (United States) | Green-backed Trogon |
| French | Trogon à queue blanche |
| French (France) | Trogon à queue blanche |
| German | Grünmanteltrogon |
| Japanese | ハグロキヌバネドリ |
| Norwegian | grønnryggtrogon |
| Polish | trogon białosterny |
| Portuguese (Brazil) | surucuá-de-barriga-amarela |
| Portuguese (Portugal) | Surucuá-de-barriga-amarela |
| Russian | Зеленохвостый трогон |
| Serbian | Zelenoleđi trogon |
| Slovak | trogón bielochvostý |
| Spanish | Trogón Dorsiverde |
| Spanish (Ecuador) | Trogón Dorsiverde (Coliblanco Amazónico) |
| Spanish (Peru) | Trogón de Espalda Verde |
| Spanish (Spain) | Trogón dorsiverde |
| Spanish (Venezuela) | Sorocuá Cola Blanca |
| Swedish | grönryggig trogon |
| Turkish | Yeşil Sırtlı Trogon |
| Ukrainian | Трогон синьоволий |
Trogon viridis Linnaeus, 1766
Definitions
- TROGON
- viridis
The Key to Scientific Names
Legend Overview
Introduction
One of the yellow-bellied trogons, the Green-backed Trogon, Trogon viridis, is widely found in Amazonia with disjunct populations in southeastern coastal Brazil and on the island of Trinidad. Until recently, these populations were classified as subspecies of White-tailed Trogon, though mitochondrial gene analysis and differences in voice and plumage served as evidence in the acceptance of this as a distinct species.
In addition to having a orange-yellow belly and under tail-coverts, males have black cheeks and throat with the rest of the head, neck, and upper chest black with iridescent purplish-blue. They have a pale blue orbital ring and a bluish white bill. The back is an iridescent green shifting to purplish-blue on rump and the upper tail is a bronze green, narrowly tipped with black. The white tips on the undertail are more extensive on the outer web giving the tail white edges with a dark center. Females are duller in color than males as the green and blue tones found in males are replaced by gray. Similar to White-tailed Trogons, the Green-backed Trogon has a very broad habitat range, frequently seen in second growth, forest edge as well as humid and dry forests up to 1,000 meters. The calls are a series of about 15-20 rapid but evenly spaced cow notes and differ from White-tailed Trogon whose call accelerates and often becomes louder towards the end. Nests in arboreal termitaria with both sexes excavating the nest which usually contains 2-3 eggs.
Field Identification
25–28 cm; male 77–99 g, female 74–107 g. Male with blue bill, pale blue orbital ring; head and breast bluish black, belly to undertail-coverts bright yellow; upperparts metallic green, with wingpanel finely vermiculated black and white (appearing grey at distance); primaries whitish on outer webs; relatively short tail violet and tipped black above, almost entirely white below owing to distal white on feathers. Differs from T. violaceus in blue orbital ring, deeper blue-black head and breast, no narrow white breastband, much less black on undertail. Female duller than male, without green and blue tones; head and upperparts dark grey, with belly to undertail-coverts yellow, faintly vermiculated smaller wing-coverts, outer tail feathers broadly tipped white. Juvenile is like adult female, but young male has some greenish-bronze gloss on upperparts and central rectrices, and both sexes have more extensive white on outer rectrices and buff spots on outer webs of smaller wing-coverts and some flight feathers.
Systematics History
Editor's Note: This article requires further editing work to merge existing content into the appropriate Subspecies sections. Please bear with us while this update takes place.
Closely related to T. melanocephalus, T. citreolus and T. bairdii; sometimes considered conspecific with T. bairdii. Until recently treated as conspecific with T. chionurus. Birds from SE Brazil described as race melanopterus, but individual variation, as well as overlap in biometrics with populations elsewhere in species’ range, render separation doubtful. Species sometimes erroneously listed as T. strigilatus; this name was applied to female in same publication as first use of viridis, but present name was the one adopted by First Reviser. Monotypic.Subspecies
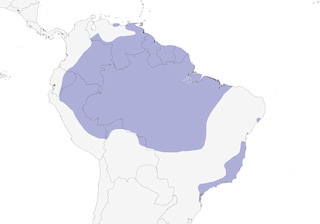
Distribution
E of Andes from Colombia S to Peru and N Bolivia, and E to the Guianas, Trinidad and E Brazil (to Maranhão and Mato Grosso; also Alagoas, and Bahia to São Paulo).
Habitat
Usually found in canopy and subcanopy of humid and wet lowland and foothill forest (both primary and selectively logged), forest edge, clearings, plantations, second-growth woodland, gallery forest and, notably, younger growth; in Colombia, occurs up to 1200 m E of Andes; in Venezuela, up to 800 m N of R Orinoco and up to 1300 m S of it, and 750 m in N Bolivia and E Peru (although it reaches 1350 m locally in the latter country). In Surinam occupies forested sand ridges and coffee plantations on coast, also occurring in savanna forest as well as interior forest; although absent from high mangroves (Avicennia) in Surinam, in neighbouring French Guiana enters swamp-forest and old mangroves. In Brazil, humid or dry forest in both lowlands and mountains up to 950 m; in Serra do Mar sometimes occurs alongside T. surrucura and T. rufus. Prefers interior of transition forest and swamp-forest in W Amazonia, while in E Amazonia the species also ranges into gallery forest in the Cerrado biome and is occasionally recorded in igapó (seasonally flooded) forest. Increases in numbers in logged forest.
Movement
Apparently sedentary.
Diet and Foraging
Fruits and berries, including Melastomaceae, Myrtaceae, Sapotaceae and Anonaceae; also beetles (lampirids), Orthoptera (including grasshoppers), large green caterpillars and other lepidopteran larvae, large ants, stick-insects, lizards (teids). Reportedly more heavily biased towards fruit than diets of smaller Trogon species, although stomach contents of 29 specimens contained arthropods alone (n = 12), arthropods and fruit (n = 6) and only fruit (n = 11) (1). Joins mixed-species flocks, but in French Guiana (where 14 foragng bouts observed in 86 minutes of continual observation) 91% of 67 observations of foraging behaviour involved solitary individuals in the canopy, and the other 9% involved birds that had joined flocks of birds in the understorey; in E Peru, 74% of observations involved birds foraging 12–25 m above ground in the lower to mid-canopy, 14% at 7–12 m up, 7% were of birds foraging below 7 m above ground, and just 5% at 25–40 m above ground in the upper canopy and emergents.
Sounds and Vocal Behavior
Song a series of up to 16 brisk “kyoh” or “cow” notes, usually becoming louder near end, sometimes with rallentando; also low purr and single “chuck” and nasal, scolding “kwa...kwa...kwo-kwo-kwo-kwo-kwo”. In Venezuela, apparently gives both fast and slow songs, the latter at a rate of c. 2·5 notes/second, the former at c. 4 notes/second and resembling that of T. violaceus, typically starting hesitantly and then gaining speed and apparent ‘confidence’; the slow song is apparently mainly heard in areas of overlap with T. violaceus, but both songs have been heard in areas where the latter does not occur (2).
Breeding
Individuals in breeding condition in Feb–Apr in Venezuela; Jun in E Colombia; excavating in Jul in E Ecuador (3); Feb–Jul in Surinam, but pairs seen excavating in Aug and at nest in Dec; Mar, May and Jul in Trinidad; Aug–Sept and Dec–Mar in French Guiana; Jan and Mar in Brazil, with copulation mid-Sept in S Brazil. Nest in arboreal termitarium, sometimes in tree hole (at least in Trinidad), often in quite open situation at edge of forest clearing, but commonly 10–20 m from ground, once c. 40 m (3), and recorded as low as 2 m. Eggs 2–3, glossy white, size 28·1–34·5 mm × 22·5–25·5 mm (4). Incubation and fledging periods unrecorded.
Conservation Status
Not globally threatened. Locally fairly common. Common in Colombia, where along with T. melanurus the most numerous trogon E of Andes; In Surinam the commonest trogon in coastal region; in French Guiana, S Venezuela and adjacent N Brazil the commonest trogon anywhere; common in Trinidad. Common in tropical lowlands in Peru, but less so above 750 m (N Peru); common at Tambopata. Present in Bolivia in Amboró and Noel Kempff Mercado National Parks. In Brazil, common in Amazonia, and present in Jaú National Park, Amazonas, and Tapajós National Park, Pará; in SE Brazil present in Sooretama Biological Reserve, Espírito Santo.
- Year-round
- Migration
- Breeding
- Non-Breeding





























